Truths:
1. Most of the Sun’s radiation that gets to the Earth’s lower atmosphere passes through substantially unabsorbed.
2. Most of the radiation is then absorbed on contact with the Earth’s surface. This includes the majority water and the minority land.
3. Most of the Earth’s surface is either water or moist vegetation.
Most of the radiation from the sun is converted to infrared wavelengths at or near the surface.
The water molecules absorb the infrared radiation causing increased vibration within the individual water molecules. This is converted into translational energy during intermolecular collisions.
Water is an unusual compound. Its molecular weight (18) is half that of nitrogen (28) and less than half oxygen (32). Water should by all rights be a gas.
The reason water is liquid or ice normally, is that water molecules are naturally attracted to each other and form large aggregates which are substantially heavier than air.
When liquid water absorbs infrared radiation or is otherwise stimulated it vibrates more quickly and more intensely. This breaks down that tendency to aggregate.
In fact, in order for an associated water molecule to break free and escape into the air, a specific amount of energy must be absorbed. This is called the Latent Heat of Vaporization.
In fact, this is a very large amount of energy as anyone who has boiled water knows.
It takes 1 calorie of heat to raise the temperature of liquid water by 1 Celsius degree.
It take 539 calories to change one gram of water to steam.
Phase changesTransitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat. If heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization ) would lead to plateaus in the temperature vs time graph. The graph below presumes that the pressure is one standard atmosphere. |
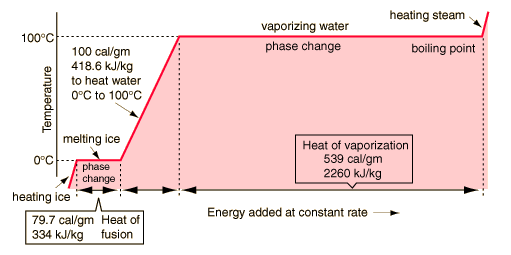
A more complete explanation of the above
All of the energy (539 cal/gm) must be lost by exchange or radiation in order for the steam to condense.
This is THE ESSENSE of the GREENHOUSE EFFECT.
Enormous amounts of energy (principally translational and vibrational) are carried from the surface into the atmosphere by fast moving free or loosely associated water molecules.
Collisions between water molecules and the majority nitrogen and oxygen molecules transfer the energy to the greater atmosphere. As the energy level of the water molecules diminishes, the probability that water molecules will reaggregate increases. This leads to condensation and has the effect of transferring that 539 calories per gram to the rest of the atmosphere.
Now for the Kicker!
Carbon dioxide does NOT form aggregates. It is not lighter than air and thus does not rise quickly. There is no phase change when carbon dioxide enters the atmosphere. Carbon dioxide carries less than half the heat per molecule compared to water.
One gram of Carbon Dioxide heated at the surface by incident sunlight carries (2 * 539 = 1078) 1078 times less energy into the atmosphere than one gram of water.
Carbon dioxide represents 0.0387 % of the atmosphere. Water in the lower atmosphere represents 1% to 4% or 25 to 100 times the amount of carbon dioxide.
Combining the two statements above, Water is (25 * 1078 = 27,175) to (100 * 1078 = 108,700) times more responsible for greenhouse effect than carbon dioxide.


10 comments:
This should be the end of the CO2 vilification! Can't argue the facts.
Nice blog
BillM wrote:
> This completely misses the
> point - water's effect is
> greater than 27000 times
> see reference.
Do you honestly think you know something about a major scientific point that experts have somehow missed in the last 200 yrs?
If so, why haven't you published this -- you would instantly win a Nobel Prize.
To David:
Actually, I don't think the hydrological cycle has been a mystery all these years. The problem is that the current atmospheric models do not attempt to understand this cycle. They just put in a feedback constant for water.
The physics I describe have been common knowledge for hundreds of years. If you doubt the effect of the latent heat of vaporization, how do you explain how long you must boil a pot of water for at very high heat to evaporate it all?
How do you explain that air blowing across your hand at 100 deg Celsius will feel hot but would take a long time to burn you. Try the same thin with steam at that temperature and the scalding you get will lead to immediate sever burns.
Virtually all the energy in thunderstorms, hurricanes, and tornadoes are produced directly from this evaporative cycle. A recent study even showed that the lightning in large storms produces antimatter - that takes a LOT of energy.
By the way, I have been studying this stuff a long time. I am a pilot. I have spent a lot of time in this atmosphere.
The AGW crowd has just about everything wrong. They attempt to find causation in correlation. Al Gore showed a chart showing temperature and Carbon Dioxide levels correlated over millennia. However, he scale the timeline on his chart to hide the fact that the CO2 levels trailed the temperature by more that 800 years. That's hardly a scientific approach.
The folks who try to tell you it's all about the 'Greenhouse Effect' are looking at a microscopic effect in the lower atmosphere. They say that it is warm on a cloudy night because the infrared radiation is reflected back from the clouds. Actually, the water in those clouds is a major absorber of that infrared.
The real answer here is that the moisture on the surface evaporated during the day and carried all that (539 cal/gm) energy into the lower atmosphere. As the water molecules rose they transferred some energy to the air molecules causing warming. At dusk the sun's radiance diminishes and the cycle shift to condensation over evaporation this give back that 539 cal/gm to the atmosphere keeping it relatively warm. Atmospheric water vapor acts like a huge capacitor.
Obviously areas like the poles and deserts where this cycle is minimal cool very quickly when the sun goes down. Ever been in Vegas in the winter when the sun drops behind the mountains? It can go from very comfortable to freezing while walking a couple of blocks.
BillM wrote:
> The problem is that the
> current atmospheric models
> do not attempt to understand
> this cycle. They just put
> in a feedback constant for water.
That is certainly not my understanding -- can you please refer me to scientific documents that demonstrate this?
Here is a GCM that is particularly transparent: NASA GISS's GCM Model E: Model Description and Reference Manual --http://www.giss.nasa.gov/tools/modelE/modelE.html
Look at the model's manual, particularly Chapter 3:
* Atmospheric model
* Dynamics
* Cloud processes
* Moist convection
* Large scale condensation
There is some serious physics here. So how can you maintain your claim that they just put in a "constant feedback" for water vapor?
To David
Certainly there is and has been an enormous amount of research and modelling of the hydrological cycle.
The problem is that it is a chaotic system. It cannot even predict weather for a week. By definition for an atmospheric model to have any predictive value it would require that it be made of a series exact forecasts each one being the input for the next in the series. If the model is correct then it should accurately predict the exact condition of the atmosphere at time X. Keep in mind that the warmists talk in terms of 0.1 to 0.2 Celsius degrees per decade.
So far, the model runs published over the last decade or so have failed miserably.
I am not claiming the ability to forecast the future. The warmists are. I am only pointing out that the concentration on CO2 is an absurdity. The actual energy flows involved in the hydrological cycle are 10’s to 100’s of thousands times greater than any radiative effects of CO2.
How did everybody miss the simple fact that water does not have to heat up to 100 C to evaporate ?? It does it everyday in my house at ambient temperatures which rarely exceed 30 C.
When I run water from the tap it "splashes" over the base of the sink. Surface tension causes these "splashes" to aggregate into drops often relatively large - up to an inch or more in size. All are usually gone by morning even when overnight temperatures are less than 10 C .
I don't suppose you have heard of the Boltzmann Distribution. Mercury thermometers measure the average kinetic energy of the molecules striking the surface. There will be almost stationary ones and others going very quickly. There will always be some achieving local escape velocity. The temperature of the local environment will determine the rate of evaporation.
At 0 degrees Kelvin (absolute 0) the particles cease moving.
The width of the Maxwell-Boltzmann distribution is well known. Almost no air molecules achieve local escape velocity, unless they're very high up in the atmosphere. None do at ground level, where the escape velocity is 11,200 m/s.
https://en.wikipedia.org/wiki/Maxwell%E2%80%93Boltzmann_distribution#/media/File:MaxwellBoltzmann-en.svg
It's impossible to reach zero Kelvin. (That's the third law of thermodynamics.) Air liquifies at 58 K at standard atmospheric pressure.
Appreciate you blogging thiis
Post a Comment