Truths:
1. Most of the Sun’s radiation that gets to the Earth’s lower atmosphere passes through substantially unabsorbed.
2. Most of the radiation is then absorbed on contact with the Earth’s surface. This includes the majority water and the minority land.
3. Most of the Earth’s surface is either water or moist vegetation.
Most of the radiation from the sun is converted to infrared wavelengths at or near the surface.
The water molecules absorb the infrared radiation causing increased vibration within the individual water molecules. This is converted into translational energy during intermolecular collisions.
Water is an unusual compound. Its molecular weight (18) is .64 times that of nitrogen (28) and .56 times that of oxygen (32). Water should by all rights be a gas.
The reason water is liquid or ice normally, is that water molecules are naturally attracted to each other and form large aggregates which are substantially heavier than air.
When liquid water absorbs infrared radiation or is otherwise stimulated it vibrates more quickly and more intensely. This breaks down that tendency to aggregate.
In fact, in order for an associated water molecule to break free and escape into the air, a specific amount of energy must be absorbed. This is called the Latent Heat of Vaporization.
In fact, this is a very large amount of energy as anyone who has boiled water knows.
It takes 1 calorie of heat to raise the temperature of one gram of liquid water by 1 Celsius degree.
It take 539 calories to change one gram of water at 100 degrees C to steam.
Phase changes
Transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat. If heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization ) would lead to plateaus in the temperature vs time graph. The graph below presumes that the pressure is one standard atmosphere.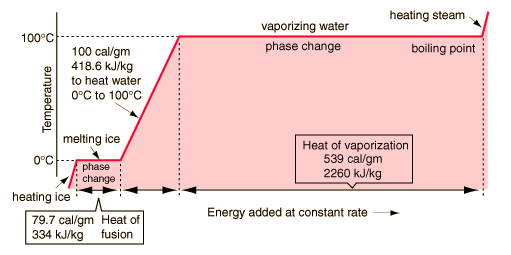
A more complete explanation of the above
All of the energy (539 cal/gm) must be lost by exchange or radiation in order for the steam to condense.
This is THE ESSENSE of the GREENHOUSE EFFECT.
Enormous amounts of energy (principally translational and vibrational) are carried from the surface into the atmosphere by fast moving free or loosely associated water molecules.
Collisions between water molecules and the majority nitrogen and oxygen molecules transfer the energy to the greater atmosphere. As the energy level of the water molecules diminishes, the probability that water molecules will reaggregate increases. This leads to condensation and has the effect of transferring that 539 calories per gram to the rest of the atmosphere.
Now for the Kicker!
Carbon dioxide does NOT form aggregates. It is not lighter than air and thus does not rise quickly. There is no phase change when carbon dioxide enters the atmosphere. Carbon dioxide carries less than half the heat per molecule compared to water.
One gram of Carbon Dioxide heated at the surface by incident sunlight carries (2 * 539 = 1078) 1078 times less energy into the atmosphere than one gram of water.
Carbon dioxide represents 0.0387 % of the atmosphere. Water in the lower atmosphere represents 1% to 4% or 25 to 100 times the amount of carbon dioxide.
Combining the two statements above, Water is (25 * 1078 = 27,175) to (100 * 1078 = 108,700) times more responsible for greenhouse effect than carbon dioxide.


2 comments:
You need to take a look at this post http://wattsupwiththat.com/2011/02/28/visualizing-the-greenhouse-effect-atmospheric-windows/#more-34919 .
Yup, I saw that. The problem is that the Infra Red radiation total energy balance effects on the lower atmosphere are miniscule.
As I point out, it is the 540 calories per gram of water heat at the surface and the consequent loss of that 540 cal/gm at altitude on condensation that transfers the heat to the atmosphere. The results from absorption and emission in the atmosphere are tiny in comparison.
A simple personal example of this heat transfer is perspiration. When your body starts to overheat whether from the air or from exercise (burn fuel in your body release heat), it takes advantage of the 540 cal/gram of water to cool your body. If it weren't for this unusual property of water, you would dehydrate and die. Same is true of a large black dog on a hot summer day. The dog pant to produce the evaporation required.
Another example is the extremely rapid cooling in the arctic and in the desert when the sun sets. There is no water left in the lower atmosphere
Post a Comment